
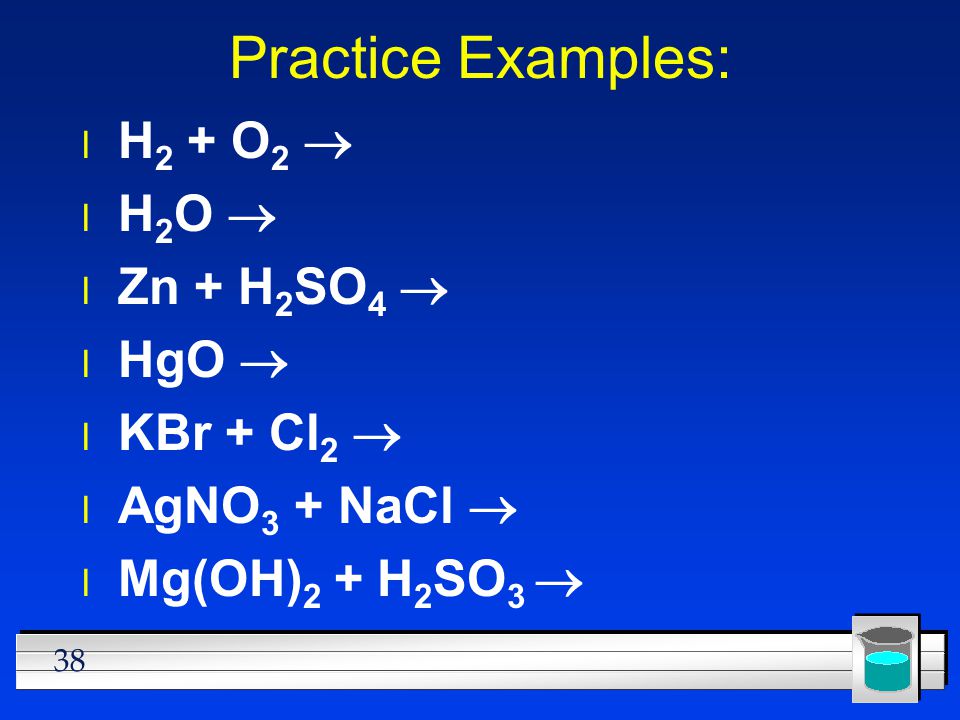

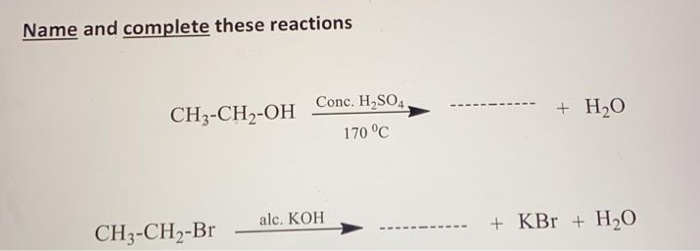
Phản ứng thế, cộng Các biện pháp xử lý sự cố tràn đổ axit H2SO4.
In Stock
$34.99
$29.99
Shipping and Returns Policy
- Deliver to United States » Shipping Policy «
- - Shipping Cost: $5.99
- - Handling time: 2-3 business days
- - Transit time: 7-10 business days
- Eligible for » Returns & Refund Policy « within 30 days from the date of delivery
Find similar items here:
kbr + h2so4
- Tạo K2SO4, HBr (nếu điều kiện phù hợp) So sánh tính axit của H2Se và H2S.
- Sản xuất H2SO4 công nghiệp
- Xử lý H2S
- Giải thích tính oxi hóa Brom
- Mỏ lưu huỳnh, quặng sunfua Ứng dụng của các hợp chất Brom khác (không phải Brom đơn chất).
- Tính chất, màu sắc, mùi của khí Khí nào được tạo ra khi KBR phản ứng với H2SO4 loãng nguội?
- Công thức và ý nghĩa Cơ chế phản ứng có vai trò gì trong việc giải thích động học của phản ứng?
- Vật liệu mới, công nghệ mới Các phương pháp thay thế cho axit sunfuric trong một số ứng dụng nhất định.
- Cơ chế đặc nóng
- Chất thay thế H2SO4
-
Next Day Delivery by USPS
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.
Our extended Christmas returns policy runs from 28th October until 5th January 2025, all items purchased online during this time can be returned for a full refund.
No reviews yet. Only logged in customers who have purchased this product may leave a review.
